According to an announcement from Caribou Biosciences, the FDA has granted advanced therapy designation in regenerative medicine for cutting-edge CAR-T therapy CB-010 for the treatment of relapsed or refractory large B-cell lymphoma, as well as fast track designation for the treatment of relapsed or refractory B-cell non-Hodgkin's lymphoma.
In the Phase I clinical trial NCT04637763, researchers evaluated CB-010 for the treatment of relapsed/refractory B-cell non-Hodgkin's lymphoma (including diffuse large B lymphoma, primary mediastinal large B-cell lymphoma and high-grade B-cell lymphoma).
CB-010 is the first clinical CD19 CAR-T cell immunotherapy to knock out PD-1. The so-called PD-1 knockdown is a genome-editing strategy used to keep CAR-T cells from prematurely failing and to enhance the durability of the therapy's anti-tumour activity.
"Receiving an Advanced Therapy in Regenerative Medicine and Fast Track designation from the FDA is recognition of the new cellular immunotherapy for the treatment of aggressive B-cell non-Hodgkin's lymphoma," said Dr. Rachel Hauwitz, president and CEO of Caribou Biosciences, in a press release.
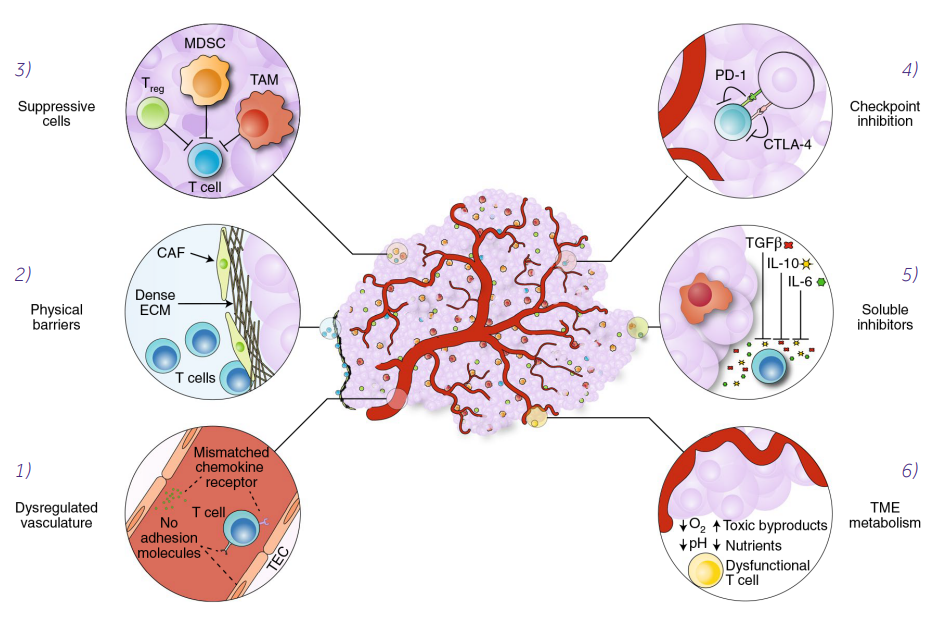
This Phase I, multicenter, open-label ANTLER clinical trial is evaluating the safety and efficacy of CB-010 in patients with relapsed/refractory B-cell non-Hodgkin's lymphoma who have received at least 2 prior lines of chemoimmunotherapy. The study consists of two parts, a dose-escalation part (testing safety) and a dose-expansion part (testing efficacy), to test and determine the assayed tolerated dose, safety and recommended dose of CB-010 for the Phase II clinical trial.
All participating patients are CD19 positive and patients who have previously received immunotherapy with CD19 cells are not eligible for enrollment.
Data presented at EHA 2022 showed that the average age of patients participating in the trial was 65 years, most were male, the average length of diagnosis was 6 years, and the average number of times patients had received systemic therapy was 3.
Results from the ANTLER trial presented at the 2022 EHA Congress showed that the safety and anti-tumour activity of CB-010 at dose level 1 (40x106 CAR-T cells) was even more encouraging, with all six patients experiencing an assorted response of complete tumour disappearance (CR).
At six months of treatment, three patients remained tumour-free, with the best sustained effect being tumour-free for 15 months.
With regard to safety, patients generally tolerated it well at a dose level of 1. Adverse reactions that have occurred during treatment include neutropenia, thrombocytopenia, anaemia, lymphopenia, elevated lactate dehydrogenase, cytokine release syndrome, elevated blood creatinine, hypocalcaemia/sodium, etc.
Grade 3 immune effector cell-related neurotoxic syndrome occurred in one patient, but resolved within 39 hours.
"By editing using our sophisticated CRISPR chRDNA genome editing technology, CB-010 can be engineered as a PD-1 knockout to limit premature CAR-T cell failure and improve the durability of anti-tumour activity," said Dr. Hauwitz. "In our Phase I trial, three of the six patients treated with CB-010 at dose level 1 maintained durable tumour disappearance at six months. This suggests that CB-010 has shown potential as a spot-on cellular immunotherapy."
