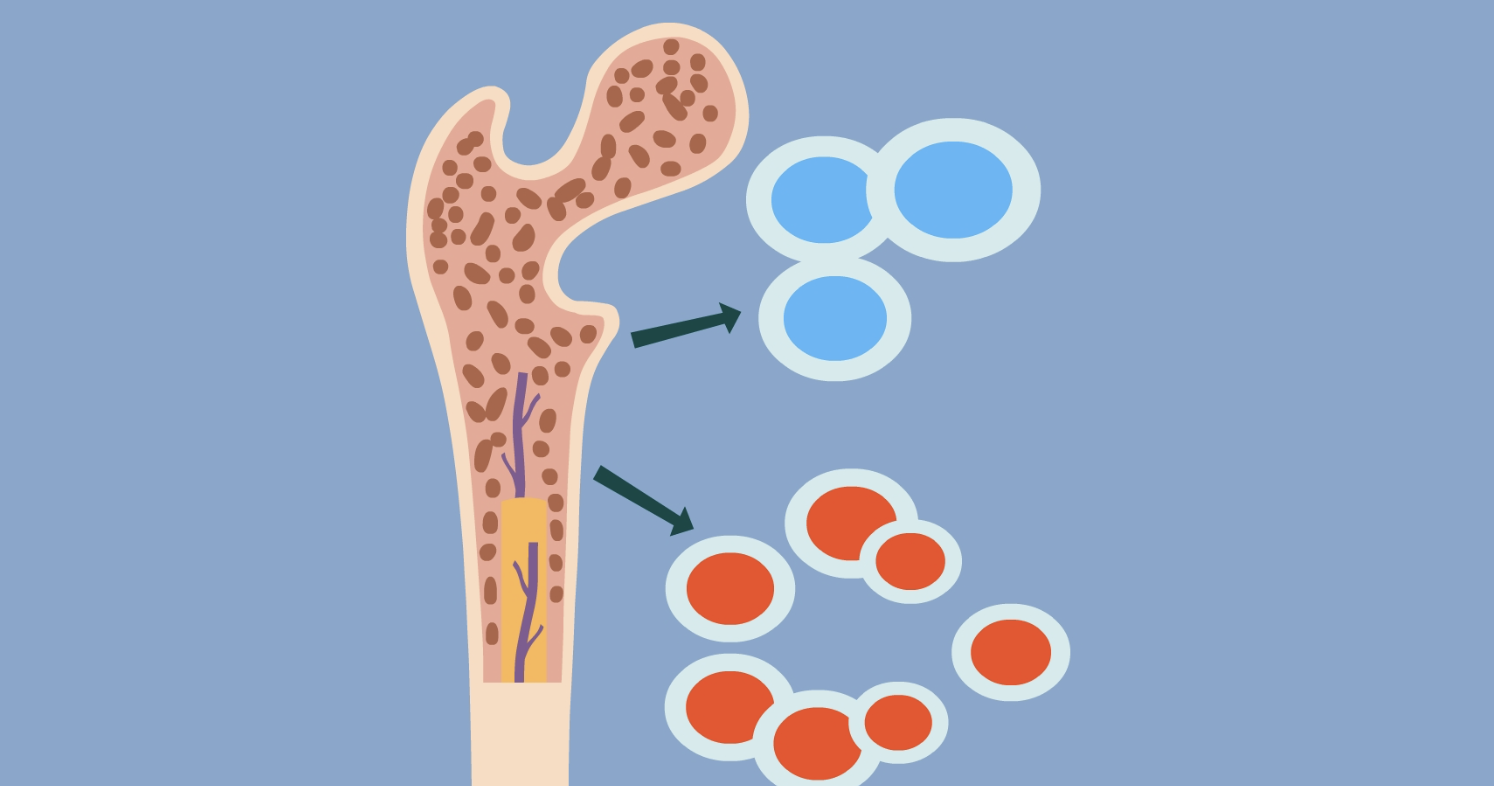
According to researchers at the Tisch Cancer Institute at the Icahn School of Medicine at Mount Sinai, a new treatment that helps the immune system kill multiple myeloma cancer cells has benefited up to 73% of patients in 2 clinical trials!

Talquetamab is 1 cutting-edge therapy known as a bispecific antibody that targets both GPRC5D and CD3. The drug activates CD3-positive T cells and induces T cells to attack GPRC5D-positive multiple myeloma. The researchers describe this treatment strategy as "bringing the army directly to the enemy and killing them".
The success of Talquetamab is applicable to patients who are resistant to all approved multiple myeloma therapies, as it uses a novel therapeutic target that is different from all other approved therapies.
Results from the Phase 1 and Phase 2 clinical trials of talquetamab were published in the New England Journal of Medicine. In one case, the recommended dose was established in the Phase 1 trial and tested in the Phase 2 trial.
The results of the Phase 2 trial were reported at the American Society of Haematology Annual Meeting on 10 December. Study participants with multiple myeloma, all of whom had previously received at least three different treatments, did not achieve durable remissions, suggesting that Talquetamab could offer new hope for patients with refractory multiple myeloma.
"About 3/4 of these patients are looking for new treatment opportunities," said Dr. Ajai Chari, director of clinical research for the Multiple Myeloma Program at the Tisch Cancer Institute and lead author of the 2 studies.
"Talquetamab has elicited substantial treatment responses in patients with relapsed or refractory multiple myeloma (the 2nd most common type of blood cancer) who have had multiple rounds of treatment. It is the first bispecific agent to target the GPRC5d protein in patients with multiple myeloma."
Typically, almost all myeloma patients who receive standard treatment experience relapse. And patients who develop resistance to multiple myeloma treatment often have a poor prognosis, so additional treatment options are urgently needed. the Talquetamab study, while an early trial designed to test therapy tolerance and find a safe dose, is also an important step forward in meeting patients' needs for additional treatment.
The Phase 1 clinical trial of Talquetamab enrolled 232 patients at multiple cancer centres worldwide between January 2018 and November 2021. Patients were tested on various doses of treatment, either intravenously or subcutaneously. In future studies, the recommended dose for patients to receive subcutaneous injections at weekly or every other week will be tested intensively.
The efficacy and safety of the Phase 1 study was further validated in the Phase 2 trial, which included 143 patients treated with the regular weekly dose and 145 patients treated with the higher bi-weekly dose.
Dr Chari said the overall remission rate in the 2 trial groups was approximately 73%. over 30% of patients in the 2 groups had complete tumour disappearance and no myeloma-specific markers were detected. Nearly 60% of patients had a "very good partial remission" or were in better shape (significant reduction in cancer, but not yet zero).
In both trial groups, side effects were relatively frequent, but usually mild and therefore tolerable.
About 3/4 of the patients experienced cytokine release syndrome, which included fever, a common feature of immunotherapy. About 60% experienced skin-related side effects such as rash, about half reported taste changes and about half reported nail disease.

Few patients (5% to 6%) discontinued Talquetamab treatment because of side effects, the researchers said, explaining that the remission rates observed in the study were higher than most currently marketed therapies, suggesting that Talquetamab holds the promise of a viable new option for and benefit from patients with multiple myeloma.