On February 6, 2023, Boogen and Sage Therapeutics jointly announced that the FDA has accepted zuranolone for the treatment of major depressive disorder ("MDD") and postpartum depression (PPD). The application has been granted priority review and the FDA has assigned a Prescription Drug User Fee Act (PDUFA) action date of August 5, 2023.
About Zuranolone
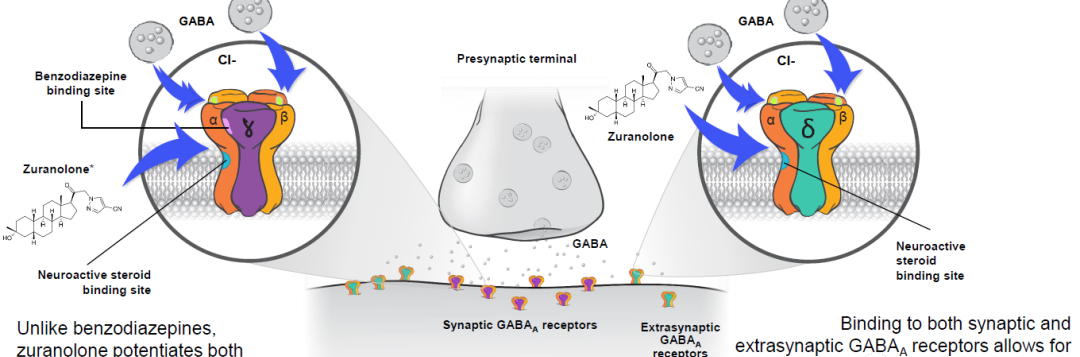
Zuranolone is a two-week, once-daily oral medication that is a neuroactive steroid (NAS) GABA-A receptor-positive modulator (PAM). It has optimized the selectivity for synaptic and extrasynaptic GABA receptors and the pharmacokinetic profile for oral administration, overcoming the significant drawback of brexanolone requiring 60h intravenous administration, with the potential advantage of being "first in class". The potential advantages of brexanolone are
In 2020, Boeing Health entered into a strategic partnership with Sage for $875 million down payment, $650 million equity, $1.6 billion mileage plus a sales commission, primarily around the development of zuranolone.
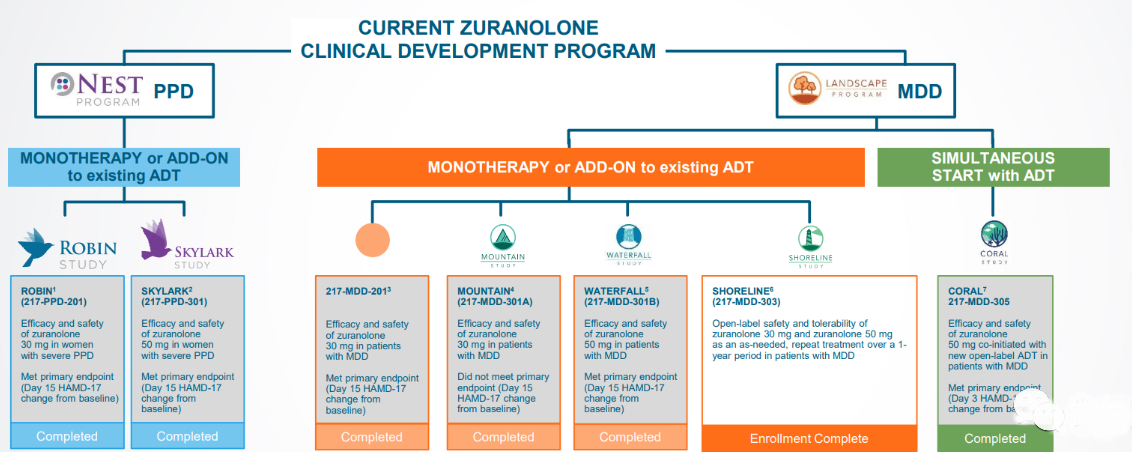
The NDA for Zuranolone is based on positive data from the LANDSCAPE and NEST development programmes, LANDSCAPE comprising five studies in adult patients with MDD (MDD-201B, MOUNTAIN, SHORELINE, WATERFALL and CORAL studies) and NEST comprising two studies in female patients with PDD (ROBIN and NEST). patients (the ROBIN and SKYLARK studies).
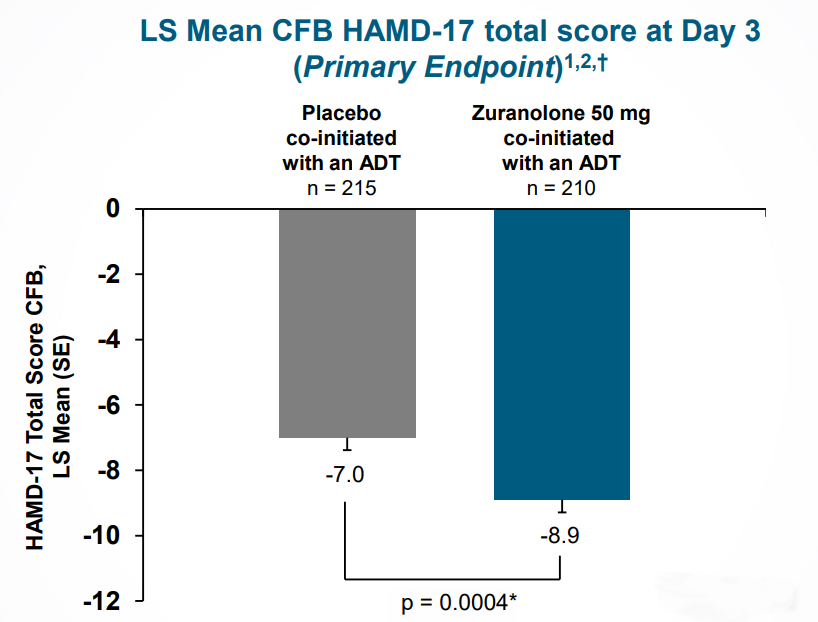
In February 2022, the CORAL study of zuranolone in patients with MDD reached its primary endpoint, demonstrating rapid efficacy against depressive symptoms, with a more pronounced effect compared to placebo observed by day 3 of the 2-week course.
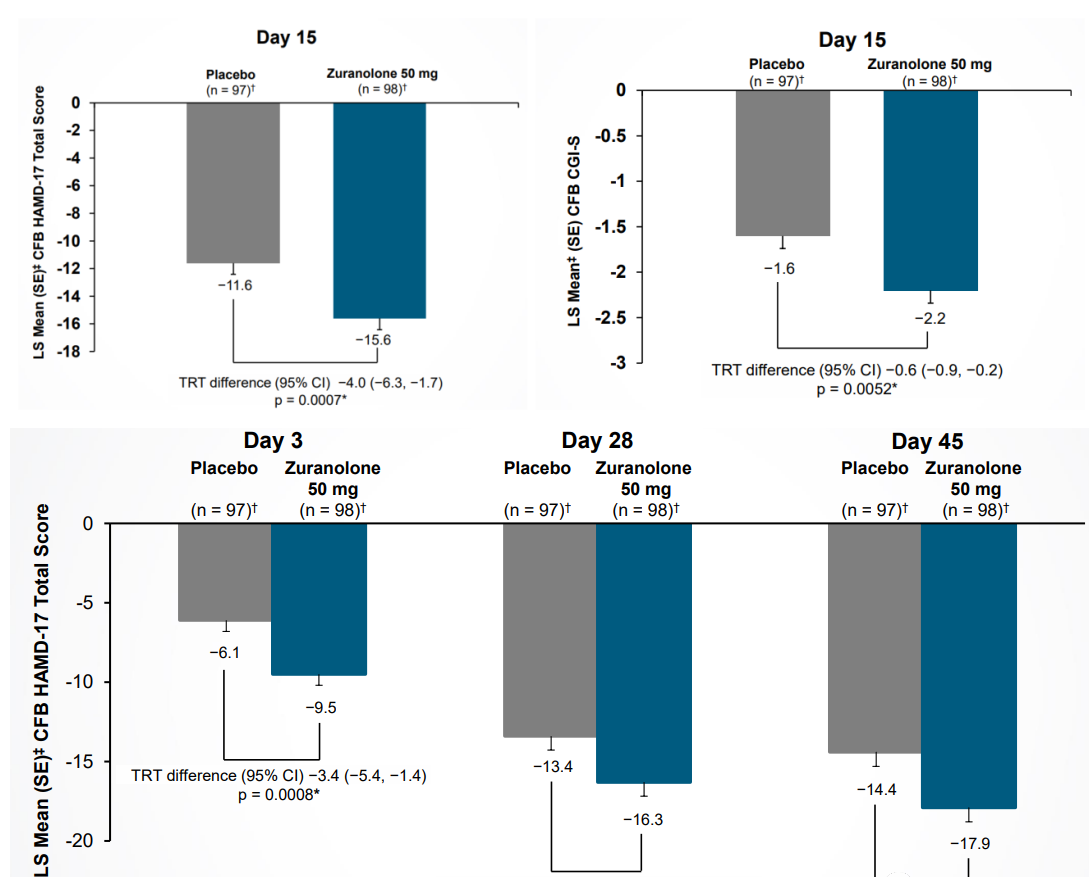
In June 2022, the SKYLARK study of zuranolone in patients with PPD showed statistically significant and clinically meaningful improvements in depressive symptoms at day 15 (primary endpoint) and days 3, 28 and 45 (key secondary endpoints), i.e. least squares (LS) mean (SE) scores on the HAMD-17 total score at day 15 for women receiving zuranolone 50 mg. (LS) mean (SE) CFB was -15.6 (0.82) compared to -11.6 (LS mean difference -4.0 points; p=0.0007) for women receiving placebo (primary endpoint). In addition, patients treated with zuranolone 50 mg had a statistically significant improvement in Clinical Global Impression Severity (CGI-S) at day 15 compared to baseline compared to placebo (zuranolone -2.2 vs. placebo -1.6, p = 0.0052).
About MDD and PDD
Depression is an affective disorder characterised by a depressed state of mind. The etiology of the disorder has not been clearly defined to date and it is generally believed that a number of factors, including biological, psychological and social circumstances, contribute to its development. MDD is characterised by widespread depressed mood for at least two weeks, low self-esteem, and a loss of interest or pleasure in enjoyable activities.
Symptoms of PPD include depressed mood, loss of interest in activities, changes in sleep patterns and appetite, decreased energy, feelings of guilt or worthlessness, poor concentration and, in some cases, suicidal thoughts, and is one of the most common complications during and after pregnancy.
According to the World Health Organisation (WHO), more than 350 million people worldwide suffer from varying degrees of depression and by 2030 depression will rank high as the number one global burden of disease, with the increase in the number of people suffering from depression driving up the demand for depression medication.
Number of people with depression in different regions of the world, 1990-2019
According to Frost & Sullivan data, the number of people suffering from depression in China reached 62 million in 2018 and is expected to grow at an annual rate of 2.6% to reach 70.5 million in 2023. With the rapid development of the antidepressant market in China, the market size in sample hospitals reached RMB 10.03 billion in 2017 and is expected to reach RMB 18.41 billion in 2022, according to Sullivan.
