According to data from a phase 2 trial, the new treatment Tovorafenib resulted in significant tumour shrinkage in 64% of patients with low-grade gliomas in children, a clinical benefit rate of 91%.
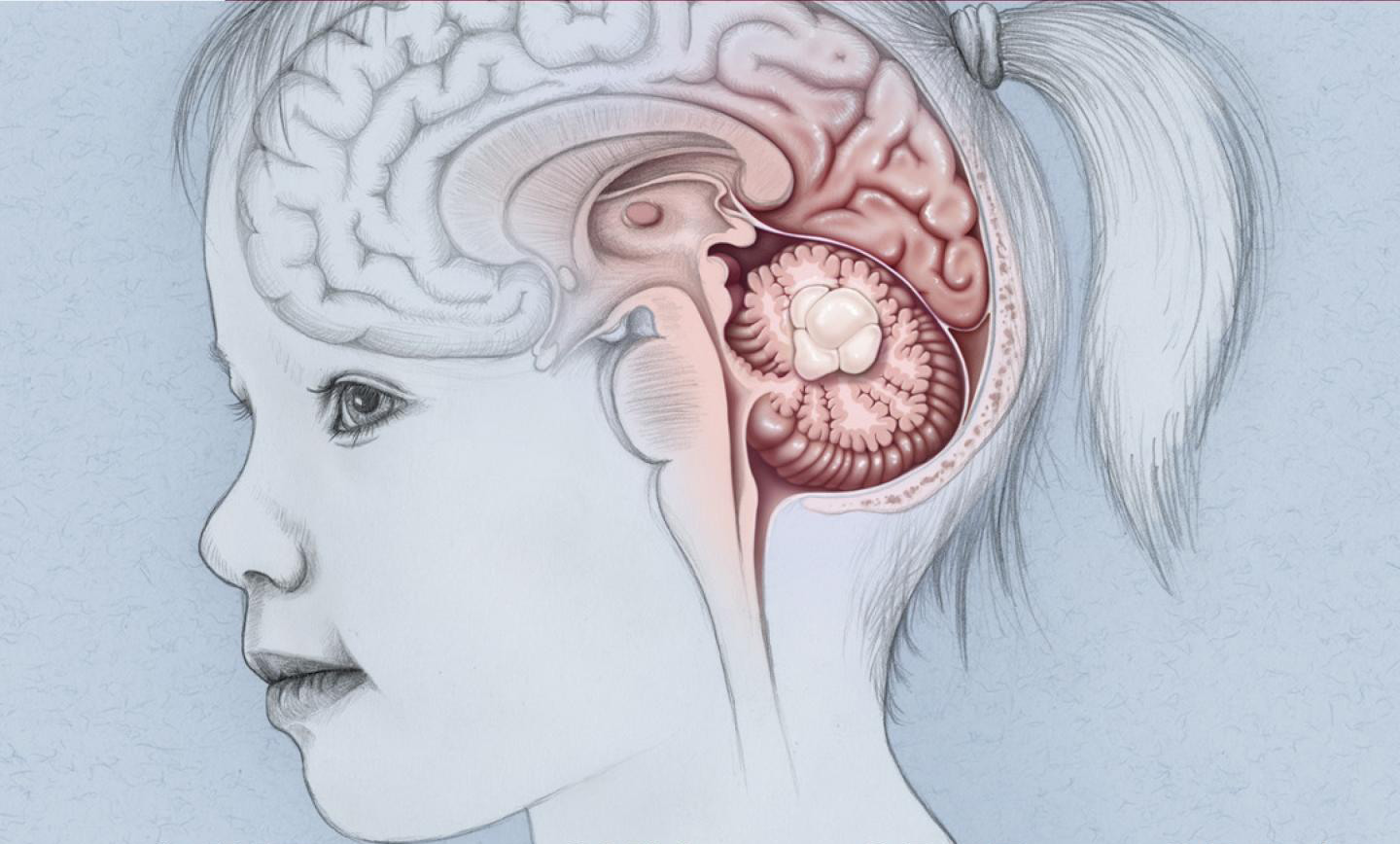
Data from a phase 2 trial in the US recently showed that in children with recurrent or progressive low-grade gliomas
Tovorafenib (DAY101) monotherapy resulted in very good anti-tumour activity.
Data from the Phase 2 FIREFLY-1 trial showed that 64% of the 69 patients enrolled on multiple treatments had a significant reduction in tumour size, with 4% of these patients having complete tumour disappearance. In addition, 28% of the patients had stable disease. This resulted in a clinical benefit rate of 91%.
Tovorafenib is a highly selective pan-RAF kinase inhibitor with brain penetration designed to target key enzymes in the MAPK signalling pathway and is currently being investigated in primary brain tumours or brain metastases from solid tumours.
Tovorafenib has been granted Breakthrough Therapy designation by the US FDA for the treatment of paediatric low-grade glioma patients harbouring RAF mutations and requiring patients with disease progression or no better treatment options on prior therapy.
Paediatric low-grade gliomas are very common childhood brain tumours for which there is no standard treatment and no approved therapy.
The FIREFLY-1 trial evaluated the efficacy of Tovorafenib monotherapy in patients aged 6 months to 25 years with recurrent or progressive low-grade gliomas.
Patients with low-grade gliomas were recruited to the trial's cohort 1 and cohort 2 and were required to have a BRAF mutation, disease progression after at least first-line systemic therapy, and at least 1 measurable lesion.
The 77 patients treated with Tovorafenib had received an average of 3 lines of therapy and 60% had been treated with at least 1 MAPK inhibitor. Of the evaluable patients, 86% carried a BRAF fusion mutation and an additional 14% had a BRAF mutation.
Safety data from 77 patients showed that Tovorafenib was well tolerated. Common drug-related adverse reactions include changes in hair colour, increased phosphocreatine kinase, anaemia, fatigue and skin rash.
The FIREFLY-1 trial is still enrolling patients at several central clinical trial centres, including the Dana-Farber Cancer Institute and Texas Children's Hospital in the US, and Great Ormond Street Children's Hospital in the UK. In addition to brain tumours, the trial's third arm is recruiting patients with other advanced solid tumours carrying activated RAS fusions.

"Based on the efficacy and safety data observed to date for Tovorafenib from the FIREFLY-1 trial population, we plan to submit a New Drug Application in the first half of 2023, which will include additional follow-up of the entire study population, and we look forward to continuing our discussions with regulatory agencies in the hopes of bringing this therapy to children in need of new treatment option for children in need of it." Jeremy Bender, Chief Executive Officer of Tovorafenib Development, said.
