On November 4, 2022, the U.S. FDA approved the launch of mivituximab soratansine (Elahere), a leading-edge antibody-coupled drug targeting folate receptor alpha (FRα), for the treatment of adult patients with platinum-resistant epithelial ovarian, fallopian tube or primary peritoneal cancer who have received one to three systemic treatment regimens and are folate receptor alpha (FRα)-positive.
Ovarian cancer has long been known as the "king of gynaecological tumours", with a higher incidence rate and the highest death rate among gynaecological tumours. For a long time, the drugs available to ovarian cancer patients have been limited. In particular, ovarian cancer patients who are resistant to platinum-based chemotherapy have a significant unmet clinical need, as follow-up treatment is difficult and survival is relatively short, and the last drug approval for these patients was in 2014.
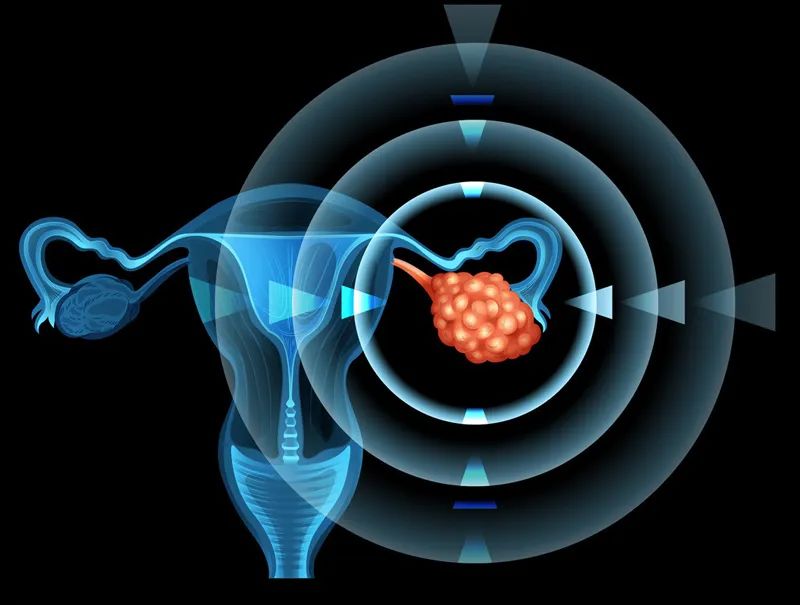
"We understand that Elahere, an antibody-coupled drug targeting folate receptor alpha (FRα), has shown very impressive anticancer activity when used alone to treat FRα-positive platinum-resistant ovarian cancer. FDA approval of the drug for marketing is therefore a major step forward for ovarian cancer patients," said Dr. Ursula A. Matulonis, chief of the Division of Gynecologic Oncology at the Dana-Farber Cancer Institute and lead author and co-principal investigator of the study, in an interview.
The marketing approval for Elahere was based on the results of the Phase III SORAYA trial led by the Dana-Farber Cancer Institute. The study showed that of 105 enrolled patients with high-grade plasma ovarian cancer resistant to prior platinum-based chemotherapy, all had received prior bevacizumab, 51% had received 3rd-line therapy, and 48% had received a PARP inhibitor.
Not only that, 59% of these patients had stage III cancer at diagnosis and 38% had stage IV disease at diagnosis, so the vast majority of patients were severely ill with intermediate to advanced disease and more than half had multi-drug resistance, which was dismal.
When it came to a mean follow-up of 13.4 months, 32.4% of patients showed definite tumour remission, i.e.: 5 patients had complete tumour disappearance and 29 patients had substantial tumour shrinkage, with a mean duration of tumour remission of 6.9 months. The drug was also well tolerated in terms of safety, with common adverse treatment times including blurred vision, keratoconus and nausea.
"The SORAYA trial demonstrated that Elahere monotherapy in patients with platinum-resistant ovarian cancer with high FRα triggered high tumour remissions, durable treatment effects and was well tolerated and safe. We observed anti-tumour activity regardless of previous bevacizumab receipt, multiple treatments, or PARP inhibitors. Given the chronic lack of effective treatments and poor prognosis for patients with this type of ovarian cancer, the findings highlight the potential of Elahere to become the standard of care for this difficult-to-treat group of ovarian cancer patients," concluded the study authors in the results published in the Journal of Clinical Oncology.
Dr Matulonis added that the approval of Elahere is crucial for patients with FRα-positive platinum-resistant ovarian cancer, who have limited treatment options and very difficult-to-treat disease, and that Elahere will be an important new cutting-edge drug in clinicians' arsenal.
