Data from a phase 1 trial recently showed that Ubamatamab monotherapy delivered promising efficacy and a good safety profile in patients with ovarian cancer who had previously received extensive treatment.
The results of the study are being presented at the annual meeting of the European Society of Medical Oncology in 2022.
The results of the study showed that of the 42 patients treated with at least one dose of Ubamatamab at 20 mg or higher, 14.3% had substantial tumour shrinkage and 57.1% had controlled disease without further progression. The treatment effect lasted for an average of 12.2 months. In addition, 23.8% of patients showed improvement in CA-125 levels.
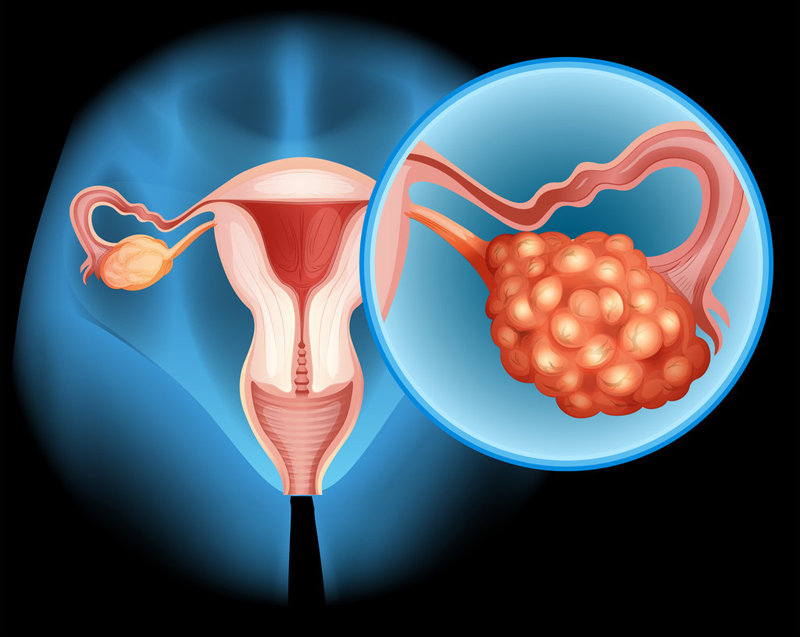
Ubamatamab is a human bispecific antibody that links MUC16 on the surface of cancer cells to CD 3 expressing T cells to promote T cell activation and release of cytotoxicity to kill cancer cells. MUC16 is expressed in approximately 80% to 90% of ovarian cancer patients, according to the researchers.
Preclinical mouse trials have shown that Ubamatamab, in combination with human immune cells, has anti-tumour activity against MUC16-expressing ovarian tumour cells and malignant ascites in the peritoneum.
This phase 1 human trial is still ongoing and involves the Memorial Sloan Kettering Cancer Center, Dana-Farber Cancer Institute and Massachusetts General Hospital in the United States.
The study enrolled patients with recurrent advanced epithelial ovarian, primary peritoneal or fallopian tube cancer who had received at least 1 line of platinum-containing chemotherapy and whose CA-125 was at least 2 times above the upper limit of normal.

The mean age of patients enrolled so far is 61 years and they have received a mean of 4.5 lines of previous treatment. Histological subtypes included high-grade plasmacytoma, clear cell carcinoma, high-grade endometrioid carcinoma, and low-grade plasmacytoma. Serum CA-125 at enrollment averaged 709 U/mL. In addition, 33% of patients had visceral metastases and 58% had >75% PS2+ immunohistochemical staining.
Other data results showed that 20.7% of patients with visceral metastases treated with at least 20 mg Ubamatamab had substantial tumour shrinkage, 72.4% had controlled disease without further exacerbation and 31.0% had improved CA-125 levels.
Among patients with PS2+ immunohistochemical staining above 75%, 30.8% had substantial tumour shrinkage, 61.5% had controlled disease and 46.2% had improved CA-125 levels.

Among patients treated at any dose, all patients enrolled experienced at least 1 treatment-related adverse reaction of any grade. 65.4% of patients experienced at least 1 adverse reaction of grade 3 or greater severity, 74.4% experienced grade 1/2 cytokine release syndrome, and 1 patient experienced immune effector cell-related neurotoxic syndrome.

The investigators noted that data from the Phase 1 trial support additional studies of Ubamatamab. Ubamatamab monotherapy as well as combination therapy with Cemiplimab is also being investigated. In addition, Ubamatamab is being investigated for subcutaneous administration to alleviate side effects.
